

Quantra® Hemostasis System
- Broadest indications of any cartridge-based whole blood hemostasis analyzer(3)
- Fastest turnaround time(4-7)
- Easy-to-read, actionable results when time is critical(8,9)
- Flexibility to test in a variety of acute care settings(3,10)
- Compact, easy to use cartridge-based system(10)
The Next Generation of Viscoelastic Hemostasis Testing
- Proprietary ultrasound technology directly measures changes in viscoelastic properties of a whole blood sample(3,7,11)
- Fully automated, sealed system(1-3)
- Flexible for any space; robust to vibration, fast start-up(3)
- Quick to learn - after a short, 30-minute training, new users were able to read and interpret the results of QPlus and QStat dials displayed with 95% proficiency(8,9)
- Simple three step workflow with hands-on time of <1 minute(3)
- Run samples directly from blue-top tubes; no wait time after draw, no pipetting(3)
- Strong correlation with applicable standard laboratory tests and VET systems(5-7,12-16)
- Easy to read, actionable results screens(8,9)
- Built-in electronic Quality Controls run every 8 hours; two levels of external biological Quality Controls(3,10)
- Integrates into existing IT infrastructure(17)
- Remote access to real-time and historical results with Quantra Desktop Remote Viewer (QDRV)(18)
- Proprietary ultrasound technology directly measures changes in viscoelastic properties of a whole blood sample3,7,11
- Fully automated, sealed system1-3
- Flexible for any space; robust to vibration, fast start-up3
- Quick to learn - after a short, 30-minute training, new users were able to read and interpret the results of QPlus and QStat dials displayed with 95% proficiency8,9
- Simple three step workflow with hands-on time of <1 minute3
- Run samples directly from blue-top tubes; no wait time after draw, no pipetting3
- Strong correlation with applicable standard laboratory tests and VET systems5-7,12-16
- Easy to read, actionable results screens8,9
- Built-in electronic Quality Controls run every 8 hours; two levels of external biological Quality Controls3,10
- Integrates into existing IT infrastructure17
- Remote access to real-time and historical results with Quantra Desktop Remote Viewer (QDRV)18

Expanded Menu of Cartridge Tests for
Hemostasis Monitoring in Critical Care Settings
Expanded Menu of Cartridge Tests for Hemostasis Monitoring in Critical Care Settings

Cardiovascular and Major Orthopedic Surgery
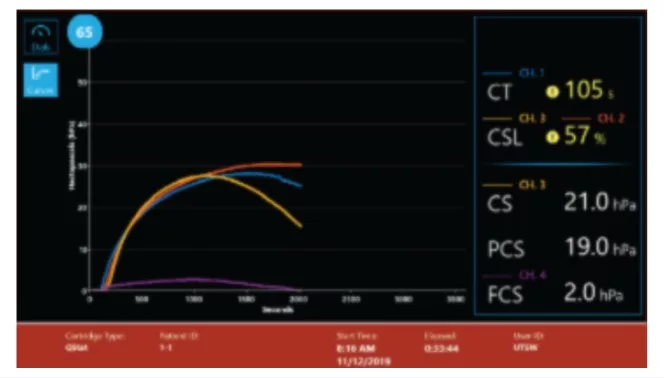
- CT: Clot time
- CTH: Clot Time with Heparinase
- CTR: Clot Time Ratio
Initial results in ~5 minutes
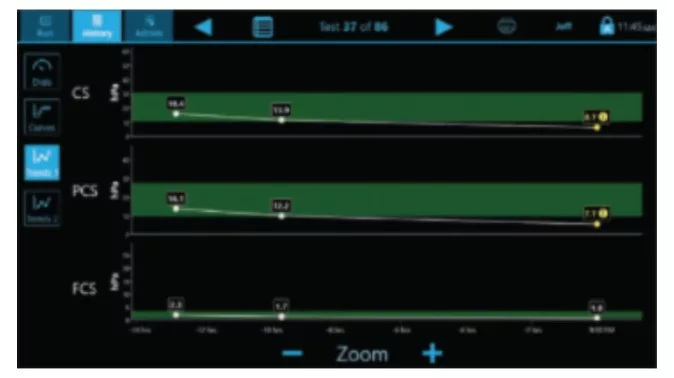
- CS: Clot Stiffness
- PCS: Platelet contribution to Clot Stiffness
- FCS: Fibrinogen contribution to Clot Stiffness
Complete results in ~12.5 minutes
- Complete results typically 15 minutes or less
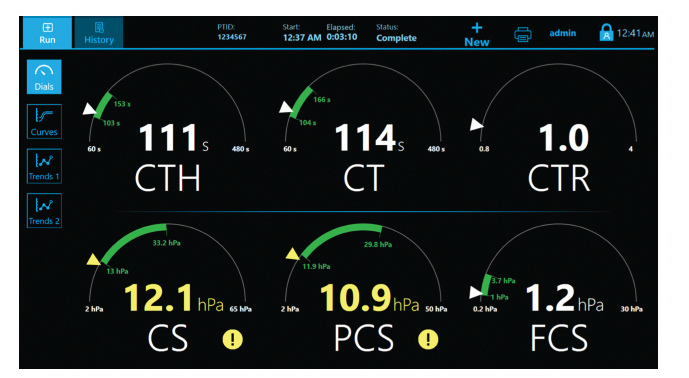
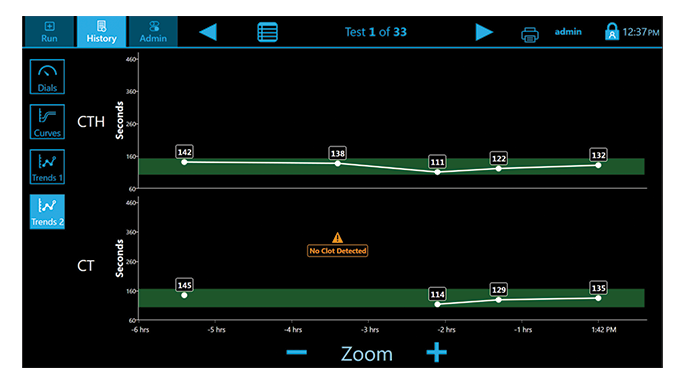
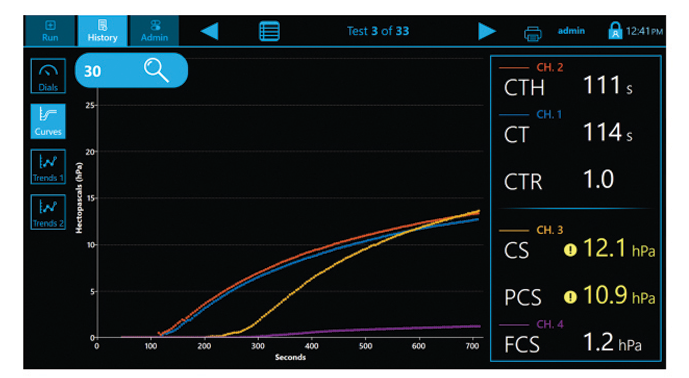
The Quantra System is currently the only VET system that directly outputs both Fibrinogen (FCS) and Platelet (PCS) Contributions to Clot Stiffness in one cartridge, helping clinicians better determine which blood products may be needed for individual patients.(1,19)
Now Available!
sthemE Qualiris by Stago is a trusted External Quality Assessment (EQA) Proficiency Testing program designed to support hemostasis and thrombosis testing worldwide. For the Quantra System and QPlus Cartridge, Qualiris offers a proficiency testing program which runs in the Spring and Fall that is both comprehensive and easy-to-use. This program enables laboratories to confidently perform external quality assessments and review detailed comparisons among a diverse global peer group.


Colored indicators and in-depth charts to quickly identify assay performance
Synthesis per level annual report1
Reporting1
Detailed per vial per parameter report
Large selection of charts available (including Youden, result dispersion, Z-score)
Youden plot for one parameter1Not concerned by accreditation

Trauma and Liver Transplantation
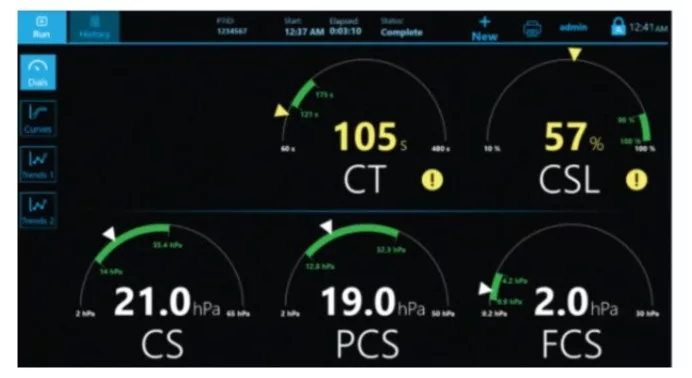
- CT: Clot Time
- CS: Clot Stiffness
- FCS: Fibrinogen contribution to Clot Stiffness
- PCS: Platelet contribution to Clot Stiffness
- CSL: Clot Stability to Lysis

Technical Specifications
| Parameter | Unit | Reportable Ranges | Healthy Reference Range |
|---|---|---|---|
| Clot Time (CT) | seconds (sec) | 60-480 | 113-164 |
| Clot time with Heparinase (CTH) - QPlus Cartridge only |
seconds (sec) | 60-480 | 103-153 |
| Clot Time Ratio (CTR) - QPlus Cartridge only |
no units (ratio) | 0.8 to 4 | Calculated parameter. CTR values > 1.4 indicate the prolongation of intrinsic CT, likely due to the influence of heparin. |
| Clot Stiffness (CS) | hectoPascals (hPa) | 2-65 | 13.0-33.2 |
| Fibrinogen Contribution to Clot Stiffness (FCS) | hectoPascals (hPa) | 0.2-30 | 1.0-3.7 |
| Platelet Contribution to Clot Stiffness (PCS) | hectoPascals (hPa) | 2-50 | 11.9-29.8 |
| Clot Stability to Lysis (CSL) - QStat cartridge only |
Percent | 10-100 | 93-100 |
Cartridge based closed system; closed tube sampling.
| 3.2% citrated venous whole blood | 3 mL |
| Undiluted venous whole blood | 2.7 mL |
| Dimensions | 36 cm (w) x 49 cm (h) x 30 cm (d) |
| Weight | 16.5 kg |
| Clearance | Top 2.5 cm, Sides 5.5 cm, Rear 5.5 cmHeat |
| Output | 75 Watts |
| Color touch screen | 22 cm x 14 cm |
| Data Input and Output | 3 USB in the rear, 1 USB in front 1- RJ45 in the rear |
| Integrated Outputs | CLSI LIS02-A2, CLSI POCT01-A2 |
| Middleware Drivers | AegisPOC® and AQURE POC®, Siemens POCCelerator® |
| LIS / HIS / Other Middleware | Developed as needed |
| Voltage | 100-240 VAC |
| Current | 1.3 A |
| Power | Input Maximum 250 Watts/Output 75 Watts |
| Frequency | 50/60 Hz |
| Power Connection Standard | 3-prong grounded; *hospital-grade cord* |
Parts and Accessories
| System Component | Ref. # |
|---|---|
| Quantra Hemostasis Analyzer | HS-001 |
| QPlus Cartridge, Kit of 10 | KT-0010 |
| Qstat Cartridge, Kit of 10 | KT-0022 |
| QPlus Control Level 1, Kit of 4 (Lyophilized 2-8 C) | KT-0024 |
| QPlus Control Level 2, Kit of 4 (Lyophilized 2-8 C) | KT-0026 |
| QStat Control Level 1, Kit of 4 (Lyophilized 2-8 C) | KT-0028 |
| QStat Control Level 2, Kit of 4 (Lyophilized 2-8 C) | KT-0038 |
| Cleaning Cartridge, Kit of 10 | KT-0012 |
| Quantra Printer | KT-0015 |
| Quantra Desktop Remote Viewer, Software | KT-0016 |
| Qualiris Proficiency Testing Kit for QPlus | KT-0048 |
Introducing our new fibrinogen POC solution

| Quantification of functional fibrinogen | Clot-based fibrinogen measurement correlated to Clause fibrinogen method |
| Sample Type | Venous whole blood in standard coagulation citrated tube (blue cap, 3.2% (0.109 M) sodium citrate) |
| Sample Volume | 15 µL |
| Precision | CV ≤ 7.0% |
| Hematocrit Range | 20% to 60% |
| Shelf Life at Room Temparature | 24 months |
| Designation | Ref.# | Packaging |
|---|---|---|
| qLabs® FIB meter | Q-3 Plus | Unit |
| eStation II docking station | MBI92 | Unit |
| qLabs® FIB Test strips | QS-18 Pro | 24 strips/box |
| qLabs® FIB Controls level 1 | QS-18-CLN | 4 x 1 mL |
| qLabs® FIB Controls level 2 | QS-18-CLP | 4 x 1 mL |
Indications: The Quantra Hemostasis System is comprised of the Quantra Hemostasis Analyzer, QPlus Cartridge, QStat Cartridge, Quantra Quality Controls (Level 1 and Level 2), Quantra Cleaning Cartridge, and Quantra Desktop Remote Viewer (QDRV) Software.
Results obtained with the Quantra System should not be the sole basis for patient diagnosis.
Rx Only.
