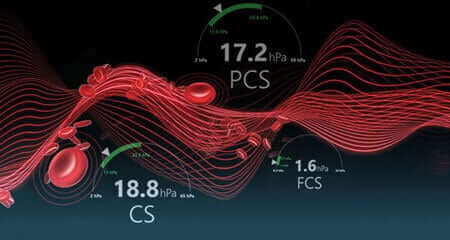

Cardiovascular and Major Orthopedic Surgery
QPlus Cartridge time to results:
- Clot time (CT)
- Heparinase clot time (CTH)
- Clot time ratio (CTR)
- Clot stiffness (CS)
- Platelet contribution to CS (PCS)
- Fibrinogen contribution to CS (FCS)
- Complete results typically 15 minutes or less
Complete results typically 15 minutes or less
INTUITIVE BY DESIGN
Understand comprehensive results at a glance.
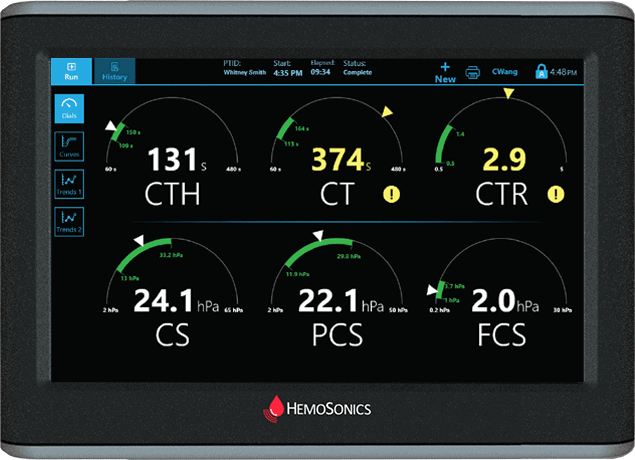
Dials Display
Intuitive display is simple to understand
Extensive parameters take the guesswork out of viscoelastic testing
Actionable outputs for treatment options
Extensive parameters take the guesswork out of viscoelastic testing
Actionable outputs for treatment options
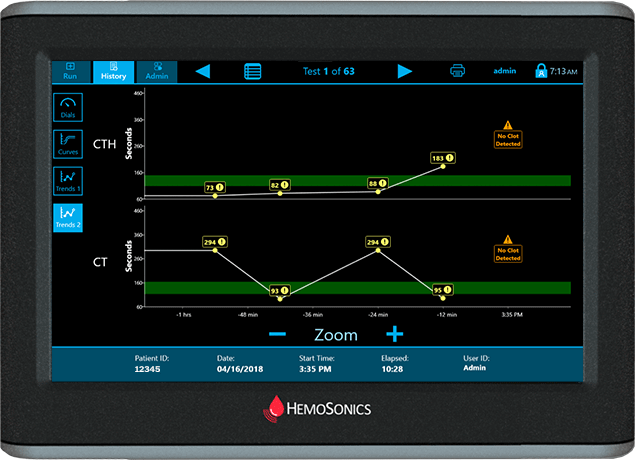
Trends Display
Easily observe trends in patient status
At a glance view of multiple patient results over time
At a glance view of multiple patient results over time
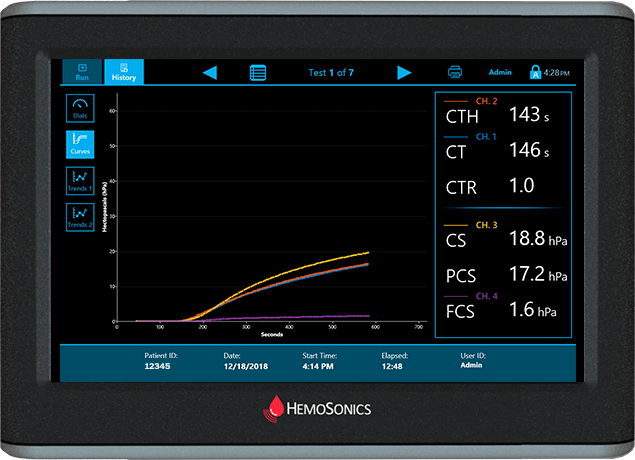
Curves Display
Traditional view of viscoelastic testing
Provides time/stiffness curve as well as numeric results
Provides time/stiffness curve as well as numeric results

Dials Display
Intuitive display is simple to understand
Extensive parameters take the guesswork out of viscoelastic testing
Actionable outputs for treatment options
Extensive parameters take the guesswork out of viscoelastic testing
Actionable outputs for treatment options

Trends Display
Easily observe trends in patient status
At a glance view of multiple patient results over time
At a glance view of multiple patient results over time

Curves Display
Traditional view of viscoelastic testing
Provides time/stiffness curve as well as numeric results
Provides time/stiffness curve as well as numeric results
The Quantra System is currently the only VET system that directly outputs both Fibrinogen (FCS) and Platelet (PCS) Contributions to Clot Stiffness in one assay, giving clinicians the opportunity to better determine which products are required by a bleeding patient.

Technical Specifications
Quantra QPlus Output Parameters
| Parameter | Unit | Reportable Ranges | Healthy Reference Ranges* |
|---|---|---|---|
| Clot Time (CT) | seconds (sec) | 60-480 | 104-166 |
| Heparinase Clot Time (CTH) | seconds (sec) | 60-480 | 103-153 |
| Clot Time Ratio (CTR) | no units (ratio) | 0.8 to 4 | Calculated parameter. CTR values >1.4 indicate the prolongation of intrinsic CT, likely due to the influence of heparin. |
| Clot Stiffness (CS) | hectoPascals (hPa) | 2-65 | 13.0-33.2 |
| Fibrinogen Contribution to Clot Stiffness (FCS) | hectoPascals (hPa) | 0.2-30 | 1.0-3.7 |
| Platelet Contribution to Clot Stiffness (PCS) | hectoPascals (hPa) | 2-50 | 11.9-29.8 |
*A reference range study was conducted for the QPlus cartridge by collecting whole blood samples from 129 healthy donors across three sites. The data were evaluated as recommended in
EP28-A3c “Defining, Establishing and Verifying Reference Intervals in the Clinical Laboratory; Approved Guideline – Third Edition” CLSI, October 2010. The reference ranges determined
from these studies are expressed as the central 95% confidence interval of the mean. It is recommended that each hospital/laboratory confirm these ranges or establish its own expected
values for the populations it serves.
Principle
Sonic Estimation of Elasticity via Resonance (SEER) Sonorheometry is an ultrasound-based
technology that measures the shear modulus of whole blood during coagulation.
Configuration
Cartridge based closed system; closed tube sampling.
Sample Volume
| 3.2% citrated venous whole blood | 3 mL |
| Undiluted venous whole blood | 2.7 mL |
Connectivity
| Data Input and Output | 3 USB in the rear, 1 USB in front 1- RJ45 ethernet connector in the rear |
| Integrated Outputs | CLSI LIS02-A2, CLSI POCT01-A2 |
| Middleware Drivers | RALS™, TELCOR®, UniPOC®, cobas® infinity, DI Data Innovations® |
| LIS Interface | CLSI LIS01-A2 Standard |
Physical Characteristics and Footprint
| Dimensions | 36 cm (w) x 49 cm (h) x 30 cm (d) |
| Weight | 16.5 kg |
| Clearance | Top 2.5 cm, Sides 5.5 cm, Rear 5.5 cmHeat |
| Display | 22 cm x 14 cm LCD Touch Screen |
Electrical Specifications
| Voltage | 100-240 VAC |
| Current | 1.3 A |
| Power | Input Maximum 250 Watts/Output 75 Watts |
| Frequency | 50/60 Hz |
| Power Connection Standard | 3-prong grounded; *hospital-grade cord* |
System Parts and Accessories
| System Component | Ref. # |
|---|---|
| Quantra Hemostasis Analyzer | HS-002 |
| QPlus® Cartridge, Kit of 10 | KT-0004 |
| QStat Cartridge, Kit of 10 | KT-0020 |
| QPlus Control Level 1, Kit of 4 (Lyophilized 2-8 C) | KT-0023 |
| QPlus Control Level 2, Kit of 4 (Lyophilized 2-8 C) | KT-0025 |
| QStat Control Level 1, Kit of 4 (Lyophilized 2-8 C) | KT-0027 |
| QStat Control Level 2, Kit of 4 (Lyophilized 2-8 C) | KT-0032 |
| Cleaning Cartridge, Kit of 10 | KT-0012 |
| Quantra Printer | KT-0015 |
| Quantra Desktop Remote Viewer, Software | KT-0039 |
- HemoSonics, LLC
- 4020 Stirrup Creek Drive, Suite 105
- Durham, NC 27703 USA
- +1 (800) 280-5589
Visit: www.HemoSonics.com Email: [email protected]
Indications: The Quantra Hemostasis System is composed of the Quantra Hemostasis Analyzer, QPlus Cartridge, QStat Cartridge, corresponding Level 1 and 2 Quality Controls,
Cleaning Cartridge, and Quantra Remote Desktop Viewer.
The Quantra QPlus System is indicated for the evaluation of blood coagulation in perioperative patients age 18 years and older to assess possible hypocoagulable and
hypercoagulable conditions in cardiovascular or major orthopedic surgeries before, during, and following the procedure.
The QStat Cartridge is indicated for the evaluation of blood coagulation and clot lysis in patients age 18 years and older to assess possible hypocoagulable and hypercoagulable
conditions in trauma and liver transplantation procedures.
Results obtained with the Quantra System should not be the sole basis for patient diagnosis. Rx Only.
HOW TO ORDER
Please contact your local sales representative for pricing and ordering information.
| System Component | Ref. # |
|---|---|
| Quantra Hemostasis Analyzer | HS-001 |
| QPlus® Cartridge, Kit of 10 | KT-0010 |
| QStat Cartridge, Kit of 10 | KT-0022 |
| QPlus Control Level 1, Kit of 4 (Lyophilized 2-8 C) | KT-0024 |
| QPlus Control Level 2, Kit of 4 (Lyophilized 2-8 C) | KT-0026 |
| System Component | Ref. # |
|---|---|
| QStat Control Level 1, Kit of 4 (Lyophilized 2-8 C) | KT-0028 |
| QStat Control Level 2, Kit of 4 (Lyophilized 2-8 C) | KT-0038 |
| Cleaning Cartridge, Kit of 10 | KT-0012 |
| Quantra Printer | KT-0015 |
| Quantra Desktop Remote Viewer, Software | KT-0016 |
| System Component | Ref. # |
|---|---|
| Quantra Hemostasis Analyzer | HS-002 |
| QPlus® Cartridge, Kit of 10 | KT-0010 |
| QStat Cartridge, Kit of 10 | KT-0022 |
| QPlus Control Level 1, Kit of 4 (Lyophilized 2-8 C) | KT-0024 |
| QPlus Control Level 2, Kit of 4 (Lyophilized 2-8 C) | KT-0026 |
| QStat Control Level 1, Kit of 4 (Lyophilized 2-8 C) | HS-0028 |
| QStat Control Level 2, Kit of 4 (Lyophilized 2-8 C) | KT-0038 |
| Cleaning Cartridge, Kit of 10 | KT-0012 |
| Quantra Printer | KT-0015 |
| Quantra Desktop Remote Viewer, Software | KT-0016 |
For a full listing of the instrument features, configurations, specifications, and parts, please contact HemoSonics, LLC.


