
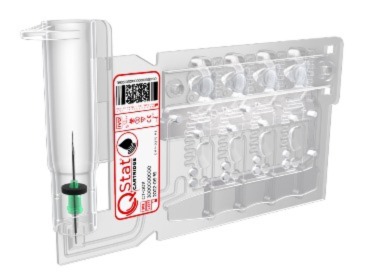
-
Trauma(1,4,9)
-
Liver Transplantation(1,9)
Trauma Liver Transplantation
The fully sealed, room-temperature stable cartridge performs multiple tests simultaneously
QStat Cartridge test parameters(9)
- CT: Clot time
- Results in ~ 5 minutes(2)
- CS: Clot stiffness
- PCS: Platelet contribution to clot stiffness
- FCS: Fibrinogen contribution to clot stiffness
- Results in ~ 12.5 minutes(2)
- CSL: Clot stability to lysis
- Lysis results in 44 ± 10 minutes on average(4)
Provides quick confirmation of clot lysis(1,4,9)
The QStat Cartridge directly compares changes in clot stiffness in both the presence and absence of tranexamic acid, automatically correcting for clot retraction caused by interaction between platelets/fibrin and the measuring device. Indicated for Trauma(1,4,9) and Liver Transplantation.(1,9)
To access Instructions for Use for this product, please log in to the Customer Portal.
INTUITIVE BY DESIGN
Understand comprehensive results at a glance.
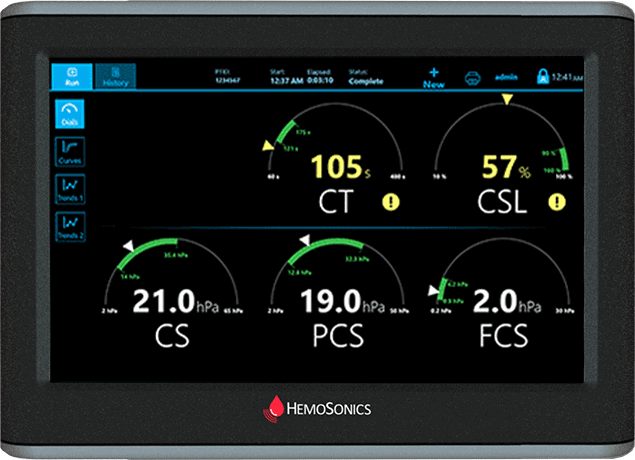
Dials Display
Intuitive display is simple to understand
Extensive parameters take the guesswork out of viscoelastic testing
Actionable outputs for treatment options
Extensive parameters take the guesswork out of viscoelastic testing
Actionable outputs for treatment options
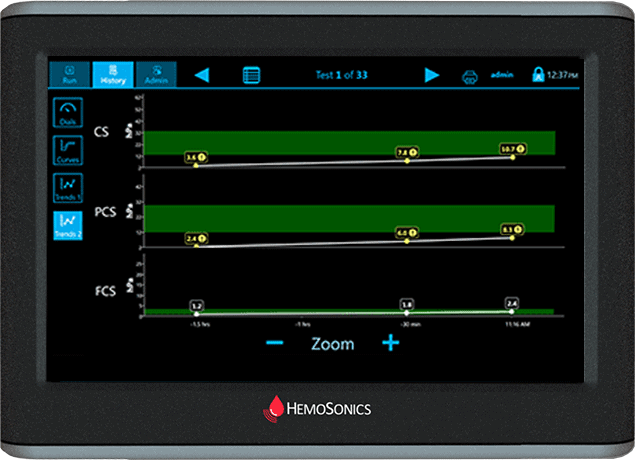
Trends Display
Easily observe trends in patient status
At a glance view of multiple patient results over time
At a glance view of multiple patient results over time
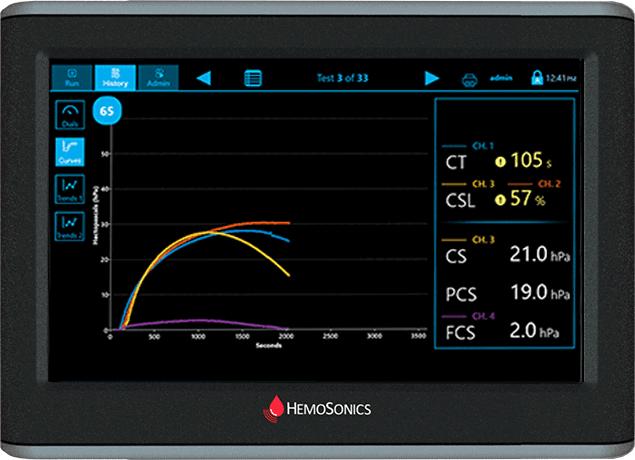
Curves Display
Traditional view of viscoelastic testing
Provides time/stiffness curve as well as numeric results
Provides time/stiffness curve as well as numeric results

Dials Display
Intuitive display is simple to understand
Extensive parameters take the guesswork out of viscoelastic testing
Actionable outputs for treatment options
Extensive parameters take the guesswork out of viscoelastic testing
Actionable outputs for treatment options

Trends Display
Easily observe trends in patient status
At a glance view of multiple patient results over time
At a glance view of multiple patient results over time

Curves Display
Traditional view of viscoelastic testing
Provides time/stiffness curve as well as numeric results
Provides time/stiffness curve as well as numeric results
HOW TO ORDER
Please contact your local sales representative for pricing and ordering information.
| System Component | Ref. # |
|---|---|
| Quantra Hemostasis Analyzer | HS-002 |
| QPlus® Cartridge, Kit of 10 | KT-0004 |
| QStat Cartridge, Kit of 10 | KT-0020 |
| QPlus Control Level 1, Kit of 4 (Lyophilized 2-8 C) | KT-0023 |
| QPlus Control Level 2, Kit of 4 (Lyophilized 2-8 C) | KT-0025 |
| System Component | Ref. # |
|---|---|
| QStat Control Level 1, Kit of 4 (Lyophilized 2-8 C) | KT-0027 |
| QStat Control Level 2, Kit of 4 (Lyophilized 2-8 C) | KT-0032 |
| Cleaning Cartridge, Kit of 10 | KT-0012 |
| Quantra Printer | KT-0015 |
| Quantra Desktop Remote Viewer, Software | KT-0039 |
| System Component | Ref. # |
|---|---|
| Quantra Hemostasis Analyzer | HS-002 |
| QPlus® Cartridge, Kit of 10 | KT-0004 |
| QStat Cartridge, Kit of 10 | KT-0020 |
| QPlus Control Level 1, Kit of 4 (Lyophilized 2-8 C) | KT-0023 |
| QPlus Control Level 2, Kit of 4 (Lyophilized 2-8 C) | KT-0025 |
| QStat Control Level 1, Kit of 4 (Lyophilized 2-8 C) | HS-0027 |
| QStat Control Level 2, Kit of 4 (Lyophilized 2-8 C) | KT-0032 |
| Cleaning Cartridge, Kit of 10 | KT-0012 |
| Quantra Printer | KT-0015 |
| Quantra Desktop Remote Viewer, Software | KT-0039 |
For a full listing of the instrument features, configurations, specifications, and parts, please contact HemoSonics, LLC.
Indications for Use
The QStat Cartridge is a multi-channel cartridge that provides semi-quantitative indications of the coagulation and clot lysis state of a 3.2% citrated venous whole blood sample using the Quantra® Hemostasis Analyzer. The QStat Cartridge includes tests to assess coagulation via the intrinsic and extrinsic pathways and includes a test with tranexamic acid to evaluate clot lysis characteristics.
The QStat Cartridge is intended for in vitro diagnostic use by trained professionals at the point-of-care and in clinical laboratories to evaluate the viscoelastic properties of whole blood by means of the following functional parameters: Clot Time (CT), Clot Stiffness (CS), Fibrinogen Contribution to Clot Stiffness (FCS), Platelet Contribution to Clot Stiffness (PCS), and Clot Stability to Lysis (CSL).
The QStat Cartridge is indicated for the evaluation of blood coagulation and clot lysis in patients age 18 years and older to assess possible hypocoagulable and hypercoagulable conditions in trauma and liver transplantation procedures.
Results obtained with the QStat Cartridge should not be the sole basis for patient diagnosis.
Rx only.
References: 1. Quantra® Hemostasis Analyzer User Manual. 2020. HemoSonics, LLC. 2. Baulig W, Akbas S, Schütt PK, et al. Comparison of the resonance sonorheometry based Quantra® system with rotational thromboelastometry ROTEM® sigma in cardiac surgery – a prospective observational study. BMC Anesthesiol. 2021;21(1):260. 3. Idowu O, Ifeanyi-Pillette I, Owusu-Agyemang P, et al. The quantra hemostasis analyzer compared to thromboelastography (TEG) in the surgical oncologic population: a prospective observational trial. J Surg Oncol. 2021;124(5):894-905. 4. Michelson EA, Cripps MW, Ray B, Winegar DA, Viola F. Initial clinical experience with the Quantra QStat System in adult trauma patients. Trauma Surg Acute Care Open. 2020;5(1):e000581. 5. Groves DS, Welsby IJ, Naik BI, et al. Multicenter evaluation of the Quantra QPlus System in adult patients undergoing major surgical procedures. Anesth Analg. 2020;130(4):899-909. 6. Winegar DA, Viola F. Is the Quantra QPlus system easy to interpret? American Association of Clinical Chemistry (AACC) Annual Scientific Meeting, Virtual Congress, December 13-17, 2020. 7. Winegar DA, Gillespie C, Sanchez-Illan M. Improving the Interpretation of Viscoelastic Test Results in the Critical Care Setting. Reader Study Abstract. American Association of Clinical Chemistry (AACC) Conference, July 2022. 8. Leadbetter NH, Givens TB, Viola F. Unique approach to quality assurance in viscoelastic testing. J Appl Lab Med. 2020;5(6):1228-1241. 9. QStat Cartridge Instructions for Use (IFU). HemoSonics, LLC.
© 2022 HemoSonics, LLC. All Rights Reserved. BR-08-12122022-US


