

Quantra® Hemostasis System
The Quantra System is the only whole blood hemostasis analyzer cleared by the FDA for use in cardiac, trauma, liver transplantation, and major orthopedic surgeries – making Quantra a trulycomprehensive solution for system-wide bleeding management(1-3).
- Broadest indications of any cartridge-based whole blood hemostasis analyzer(3)
- Fastest turnaround time(4-7)
- Easy-to-read, actionable results when time is critical(8,9)
- Flexibility to test in a variety of acute care settings(3,10)
- Compact, easy to use cartridge-based system(10)
Easily standardize across your health system to provide goal-directed therapy and help optimize blood product usage
The Next Generation of Viscoelastic Hemostasis Testing
- Proprietary ultrasound technology directly measures changes in viscoelastic properties of a whole blood sample(3,7,11)
- Fully automated, sealed system(1-3)
- Flexible for any space; robust to vibration, fast start-up(3)
- Quick to learn - after a short, 30-minute training, new users were able to read and interpret the results of QPlus and QStat dials displayed with 95% proficiency(8,9)
- Simple three step workflow with hands-on time of <1 minute(3)
- Run samples directly from blue-top tubes; no wait time after draw, no pipetting(3)
- Strong correlation with applicable standard laboratory tests and VET systems(5-7,12-16)
- Easy to read, actionable results screens(8,9)
- Built-in electronic Quality Controls run every 8 hours; two levels of external biological Quality Controls(3,10)
- Integrates into existing IT infrastructure(17)
- Remote access to real-time and historical results with Quantra Desktop Remote Viewer (QDRV)(18)
- Proprietary ultrasound technology directly measures changes in viscoelastic properties of a whole blood sample3,7,11
- Fully automated, sealed system1-3
- Flexible for any space; robust to vibration, fast start-up3
- Quick to learn - after a short, 30-minute training, new users were able to read and interpret the results of QPlus and QStat dials displayed with 95% proficiency8,9
- Simple three step workflow with hands-on time of <1 minute3
- Run samples directly from blue-top tubes; no wait time after draw, no pipetting3
- Strong correlation with applicable standard laboratory tests and VET systems5-7,12-16
- Easy to read, actionable results screens8,9
- Built-in electronic Quality Controls run every 8 hours; two levels of external biological Quality Controls3,10
- Integrates into existing IT infrastructure17
- Remote access to real-time and historical results with Quantra Desktop Remote Viewer (QDRV)18

Expanded Menu of Cartridge Tests for
Hemostasis Monitoring in Critical Care Settings
Expanded Menu of Cartridge Tests for Hemostasis Monitoring in Critical Care Settings

Cardiovascular and Major Orthopedic Surgery
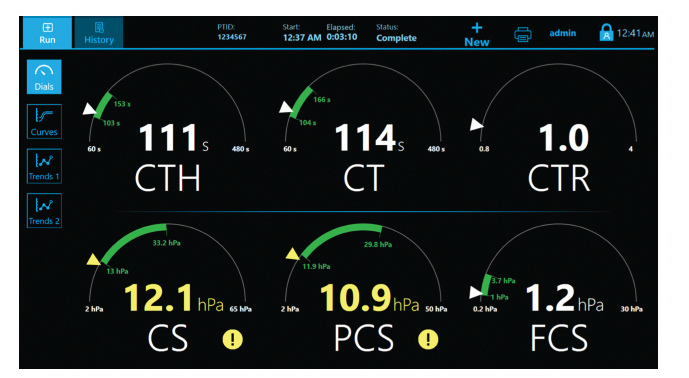
QPlus Cartridge Time Parameters(1):
- CT: Clot time
- CTH: Clot Time with Heparinase
- CTR: Clot Time Ratio
Initial results in ~5 minutes
- CS: Clot Stiffness
- PCS: Platelet contribution to Clot Stiffness
- FCS: Fibrinogen contribution to Clot Stiffness
Complete results in ~12.5 minutes
- Complete results typically 15 minutes or less
Dials
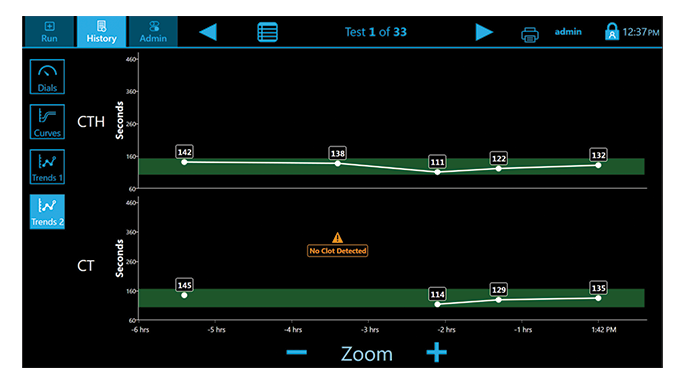
Trends
Curves
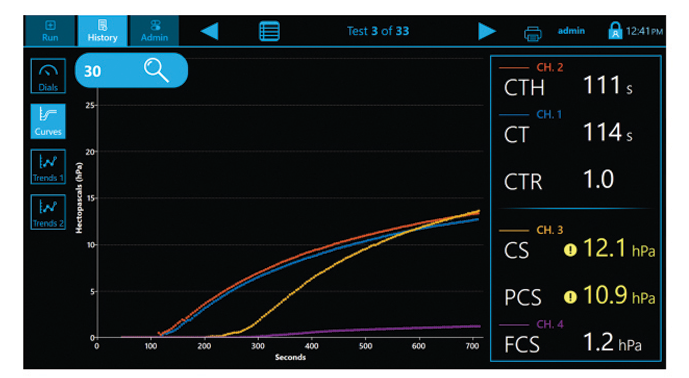
The Quantra System is currently the only VET system that directly outputs both Fibrinogen (FCS) and Platelet (PCS) Contributions to Clot Stiffness in one cartridge, helping clinicians better determine which blood products may be needed for individual patients.(1,19)

Trauma and Liver Transplantation
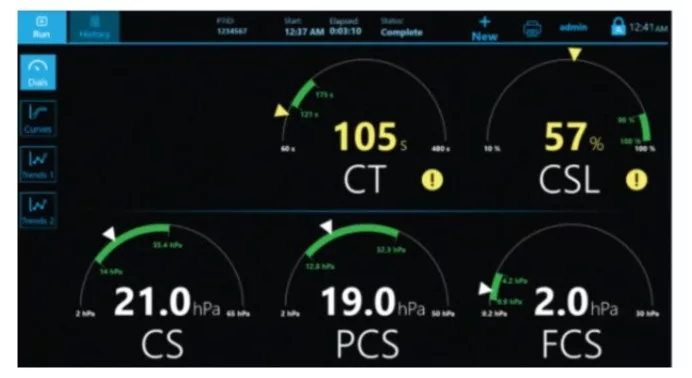
QStat Cartridge time to results(2):
- CT: Clot Time
Initial results in ~ 5 minutes
- CS: Clot Stiffness
- FCS: Fibrinogen contribution to Clot Stiffness
- PCS: Platelet contribution to Clot Stiffness
Results in ~ 12.5 minutes
- CSL: Clot Stability to Lysis
Lysis results in 25 – 60 minutes
Dials
Trends
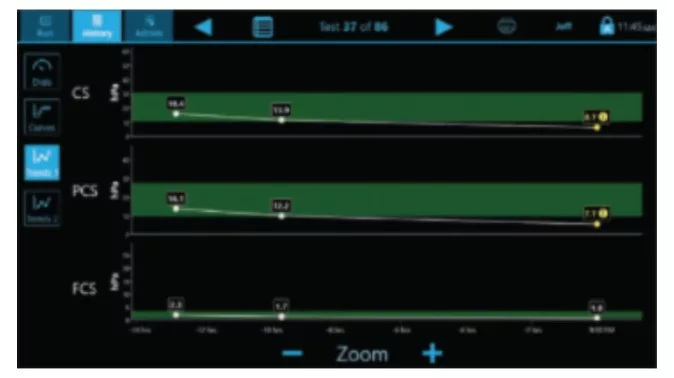
Curves
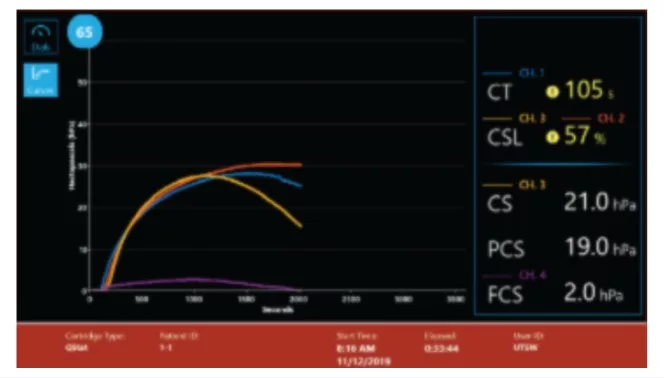
Multiple display screens present a comprehensive review of results for easy interpretation.(3)

Technical Specifications
Quantra QPlus and QStat Output Parameters
| Parameter | Unit | Reportable Ranges | Healthy Reference Range |
|---|---|---|---|
| Clot Time (CT) | seconds (sec) | 60-480 | 113-164 |
| Clot time with Heparinase (CTH) - QPlus Cartridge only |
seconds (sec) | 60-480 | 103-153 |
| Clot Time Ratio (CTR) - QPlus Cartridge only |
no units (ratio) | 0.8 to 4 | Calculated parameter. CTR values > 1.4 indicate the prolongation of intrinsic CT, likely due to the influence of heparin. |
| Clot Stiffness (CS) | hectoPascals (hPa) | 2-65 | 13.0-33.2 |
| Fibrinogen Contribution to Clot Stiffness (FCS) | hectoPascals (hPa) | 0.2-30 | 1.0-3.7 |
| Platelet Contribution to Clot Stiffness (PCS) | hectoPascals (hPa) | 2-50 | 11.9-29.8 |
| Clot Stability to Lysis (CSL) - QStat cartridge only |
Percent | 10-100 | 93-100 |
A reference range study was conducted for the QPlus cartridge by collecting whole blood samples from 129 healthy donors across three sites. The data were evaluated as recommended in EP28-A3c “Defining, Establishing and Verifying Reference Intervals in the Clinical Laboratory; Approved Guideline – Third Edition” CLSI, October 2010. The reference range for the Clot Stability to Lysis (CSL) parameter was similarly determined in a study of 43 healthy adult donors conducted at two sites in the US with the QStat Cartridge. The reference ranges determined from these studies are expressed as the central 95% confidence interval of the mean. It is recommended that each hospital/laboratory confirm these ranges or establish its own expected values for the populations it serves.
Principle
Sonic Estimation of Elasticity via Resonance (SEER) Sonorheometry is an ultrasound-based technology that measures the shear modulus of whole blood during coagulation and clot lysis.
Configuration
Cartridge based closed system; closed tube sampling.
Sample Volume
| 3.2% citrated venous whole blood | 3 mL |
| Undiluted venous whole blood | 2.7 mL |
Physical Characteristics and Footprint
| Dimensions | 36 cm (w) x 49 cm (h) x 30 cm (d) |
| Weight | 16.5 kg |
| Clearance | Top 2.5 cm, Sides 5.5 cm, Rear 5.5 cmHeat |
| Output | 75 Watts |
Display
| Color touch screen | 22 cm x 14 cm |
Connectivity
| Data Input and Output | 3 USB in the rear, 1 USB in front 1- RJ45 in the rear |
| Integrated Outputs | CLSI LIS02-A2, CLSI POCT01-A2 |
| Middleware Drivers | AegisPOC and AQURE POC, Siemens POCCelerator |
| LIS / HIS / Other Middleware | Developed as needed/td> |
Electrical Specifications
| Voltage | 100-240 VAC |
| Current | 1.3 A |
| Power | Input Maximum 250 Watts/Output 75 Watts |
| Frequency | 50/60 Hz |
| Power Connection Standard | 3-prong grounded; *hospital-grade cord* |
Parts and Accessories
| System Component | Ref. # |
|---|---|
| Quantra Hemostasis Analyzer | HS-001 |
| QPlus Cartridge, Kit of 10 | KT-0010 |
| Qstat Cartridge, Kit of 10 | KT-0022 |
| QPlus Control Level 1, Kit of 4 (Lyophilized 2-8 C) | KT-0024 |
| QPlus Control Level 2, Kit of 4 (Lyophilized 2-8 C) | KT-0026 |
| QStat Control Level 1, Kit of 4 (Lyophilized 2-8 C) | KT-0028 |
| QStat Control Level 2, Kit of 4 (Lyophilized 2-8 C) | KT-0038 |
| Cleaning Cartridge, Kit of 10 | KT-0012 |
| Quantra Printer | KT-0015 |
| Quantra Desktop Remote Viewer, Software | KT-0016 |
Introducing our new fibrinogen POC solution

qLabs® FIB
Fibrinogen Monitoring System
Fibrinogen plays a critical role in the haemostatic process and has been identified as the first factor to reach critically low levels during active bleeding such as trauma and PPH.
Stago is proud to introduce the new qLabs FIB, a point-of-care device that measures fibrinogen levels within 1 to 10 minutes. This enables rapid, goal-directed therapy, with early administration of fibrinogen supplementation in the management of critical bleeds.
Features and Specs
| Quantification of functional fibrinogen | Clot-based fibrinogen measurement correlated to Clause fibrinogen method |
| Sample Type | Venous whole blood in standard coagulation citrated tube (blue cap, 3.2% (0.109 M) sodium citrate) |
| Sample Volume | 15 µL |
| Precision | CV ≤ 7.0% |
| Hematocrit Range | 20% to 60% |
| Shelf Life at Room Temparature | 24 months |
Features and Specs
| Designation | Ref.# | Packaging |
|---|---|---|
| qLabs® FIB meter | Q-3 Plus | Unit |
| eStation II docking station | MBI92 | Unit |
| qLabs® FIB Test strips | QS-18 Pro | 24 strips/box |
| qLabs® FIB Controls level 1 | QS-18-CLN | 4 x 1 mL |
| qLabs® FIB Controls level 2 | QS-18-CLP | 4 x 1 mL |
Indications: The Quantra Hemostasis System is comprised of the Quantra Hemostasis Analyzer, QPlus Cartridge, QStat Cartridge, Quantra Quality Controls (Level 1 and Level 2), Quantra Cleaning Cartridge, and Quantra Desktop Remote Viewer (QDRV) Software.
The QPlus Cartridge is indicated for the evaluation of blood coagulation in perioperative patients age 18 years and older to assess possible hypocoagulable and hypercoagulable
conditions in cardiovascular or major orthopedic surgeries before, during, and following the procedure.
The QStat Cartridge is indicated for the evaluation of blood coagulation and clot lysis in patients age 18 years and older to assess possible hypocoagulable and hypercoagulable conditions in trauma and liver transplantation procedures
Results obtained with the Quantra System should not be the sole basis for patient diagnosis.
Rx Only.
References: 1. QPlus Cartridge Instructions for Use (IFU). HemoSonics, LLC. 2. QStat Cartridge Instructions for Use (IFU). HemoSonics, LLC. 3.Quantra® Hemostasis Analyzer User Manual. 2020. HemoSonics, LLC. 4. Baulig W, Akbas S, Schütt PK, et al. Comparison of the resonance sonorheometry based Quantra® system with rotational thromboelastometry ROTEM® sigma in cardiac surgery – a prospective observational study. BMC Anesthesiol. 2021;21(1):260. 5. Idowu O, Ifeanyi-Pillette I,Owusu-Agyemang P, et al. The quantra hemostasisanalyzer compared to thromboelastography (TEG) in the surgical oncologic population: a prospective observational trial. J Surg Oncol. 2021;124(5):894-905. 6. Michelson EA, Cripps MW, Ray B, Winegar DA, Viola F. Initial clinical experience with the Quantra QStat System in adult trauma patients. Trauma Surg Acute Care Open. 2020;5(1):e000581. 7. Groves DS, Welsby IJ, Naik BI, et al. Multicenter evaluation of the Quantra QPlus System in adult patients undergoing major surgical procedures. Anesth Analg. 2020;130(4):899-909. 8. Winegar DA, Viola F. Is the Quantra QPlus system easy to interpret? American Association of Clinical Chemistry (AACC) Annual Scientific Meeting, Virtual Congress, December 13-17, 2020. 9. Winegar DA, Gillespie C, Sanchez-Illan M. Improving the interpretation of viscoelastic test results in the critical care setting. American Association of Clinical Chemistry (AACC) Annual Scientific Meeting, July 24-28, 2022. 10. Leadbetter NH, Givens TB, Viola F. Unique approach to quality assurance in viscoelastic testing. J Appl Lab Med. 2020;5(6):1228-1241. 11. Ferrante EA, Blasier KR, Givens TB, Lloyd CA, Fischer TJ, Viola F. A novel device for the evaluation of hemostatic function in critical care settings. Anesth Analg. 2016;123(6):1372-1379. 12. DeAnda A, Levy G, Kinsky M, et al. Comparison of the Quantra QPlus System with thromboelastography in cardiac surgery. J Cardiothorac Vasc Anesth. 2021;35(4)1030-1036. 13. Naik BI, Durieux ME, Knisely A, et al. SEER sonorheometry versus rotational thromboelastometry in large volume blood loss spine surgery. Anesth Analg. 2016;123(6):1380-1389. 14. Huffmyer JL, Fernandez LG, Haghighian C, Terkawi AS, Groves DS. Comparison of SEER sonorheometry with rotational thromboelastometry and laboratory parameters in cardiac surgery. Anesth Analg. 2016;123(6):1390-1399. 15. Reynolds PS, Middleton P, McCarthy H, Spiess BD. A comparison of a new ultrasound-based whole blood viscoelastic test (SEER sonorheometry) versus thromboelastography in cardiac surgery. Anesth Analg. 2016;123(6):1400-1407. 16. Baryshnikova E, Di Dedda U, Ranucci M. A comparative study of SEER sonorheometry versus standard coagulation tests, rotational thromboelastometry, and multiple electrode aggregometry in cardiac surgery. J Cardiothorac Vasc Anesth. 2019 ;33(6):1590-1598. 17. Quantra System IT and Networking Guide. HemoSonics, LLC. 18. Quantra Desktop Remote Viewer Instructions for Use (IFU). HemoSonics, LLC. 19. Ranucci M, Baryshnikova E. Sensitivity of viscoelastic tests to platelet function. J Clin Med. 2020; 9(1):189.
© 2023 HemoSonics, LLC. All Rights Reserved. BRO-06-US 010323


