Proud to be your partner in better bleeding management
Stop the bleeding FAST.
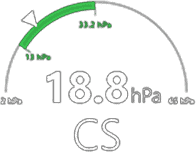

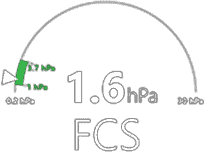
Viscoelastic testing has been demonstrated(1-3) to reduce:
Unnecessary blood
product usage
Length of stay
in hospital

Complications associated with bleeding or transfusions
Costs associated with blood product usage
Time between diagnostics and hemostatic intervention
Unnecessary blood
product usage
Length of stay
in hospital

Complications associated with bleeding or transfusions
Costs associated with blood product usage
Time between diagnostics and hemostatic intervention
Watch Quantra in Action
References: 1. Weber CF, et al. Point-of-care testing: a prospective, randomized clinical trial of efficacy in coagulopathic cardiac surgery patients. Anesthesiology. 2012 Sep;117(3):531-547. 2. Pearse BL, et al. Protocol guided bleeding management improves cardiac surgery patient outcomes. Vox Sang. 2015;109(3):267-279. 3. Haas T, et al. Reproducibility of thrombelastometry: Point-of-care versus hospital laboratory performance. Scand J Clin Lab Invest. 2012;72(4):313-317.
Our Awards
Revolutionizing VET is its own reward. but it’s always an honor to have our work recognized.
See the awards we’ve won and the organizations we have had the privilege of being acknowledged by.
Previous
Next